What could be the identities of U, V and W?
Solution
DIt is not possible to identify the ionic compound V from the data given.
For U, the choice is Zinc since it is insoluble in water. Na reacts with water to produce NaOH.
A boiling point of 58 degrees Celsius indicates that W is a liquid at room temperature. Hence SiCl4 is chosen as HCl is a gas at room temperature.
Since U=Zinc and W=SiCl4, the answer is D.
Ref: 9701/12/O/N/15 #17
A student observed the reactions when sodium chloride and sodium iodide were each reacted separately with concentrated sulfuric acid and with concentrated phosphoric acid. Some observations are recorded in the table.
Which deduction can be made from these observations?
A. Concentrated phosphoric acid is a stronger oxidising agent than concentrated sulfuric acid.
B. Concentrated phosphoric acid is a stronger oxidising agent than iodine.
C. Concentrated sulfuric acid is a stronger oxidising agent than chlorine.
D. Concentrated sulfuric acid is a stronger oxidising agent than iodine.
Solution
D
Concentrated H2SO4 and H3PO4 both react with sodium chloride and sodium iodide to form colourless acidic gases (HCl and HI respectively). Both H2SO4 and H3PO4 are weaker oxidising agents than chlorine, hence they are not able to oxidise the chloride ions in HCl.
Concentrated H2SO4 is a stronger oxidising agent than iodine, hence it is able to oxidise the iodide ions in HI to iodine gas (purple vapour).
Concentrated H3PO4 is a weaker oxidising agent than iodine, hence it is unable to oxidise the iodide ions in HI.
Ref: 9701/12/O/N/15 #24
The depletion of the ozone layer in the upper atmosphere reduces the Earth's natural protection from harmful ultraviolet radiation.
Which compound would cause the most depletion of the ozone layer?
A. CCl3F
B. CF4
C. CO2
D. SO2
Solution
A
C-Cl has the lowest bond energy, so it is the easiest bond to break. Chlorine free radicals are formed when exposed to ultraviolet radiation. (For questions like this, always look for the molecule with the most C-Cl bonds.) These chlorine free radicals will go on to destroy the ozone layer according to the following steps:
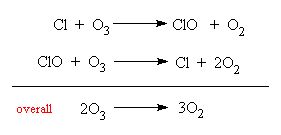
Ref: 9701/12/O/N/15 #25
Compound X has been investigated for use as an artificial sweetener.
What are the numbers of specified types of -OH groups before and after hydrolysing the two C-Cl bonds?
Solution
CThe circled blue -OH groups are secondary alcohols. The green -OH group is the primary alcohol. The red -OH group is the tertiary alcohol.
Ref: 9701/12/O/N/15 #37
Compound Q is obtained by adding H2O across the double bond in compound P.
1. P shows cis-trans isomerism.
2. Q contains two chiral centres.
3. Q is a tertiary alcohol.
Solution
C. 2 and 3 only
We highly recommend that you take a look at the explanation on optical isomers for skeletal formulae over at Chemguide.
1 is wrong because the two carbon atoms in the double bond are part of the ring structure. Hence, geometrical isomerism is "restricted" --- the groups around each carbon atom cannot be rotated to give cis-trans isomers.
Ref: 9701/12/O/N/15 #40
Compound Z is heated with concentrated acidified potassium manganate(VII). This produces an equimolar mixture of CO2 and CH3COCH2CH2CH(COCH3)CH2CO2H.
What could be the structural formula of Z?
Solution
A. 1, 2 and 3
A. 1, 2 and 3
The C=C double bonds are broken when the alkene undergoes vigorous oxidation.
Primary carbon atom with a double bond will form CO2.
Secondary carbon atom with a double bond will form carboxylic acid.
Tertiary carbon atom with a double bond will form ketone.









THANKS FOR THE GREAT WORK!!! BTW CAN I POST MY PROBLEMS?
ReplyDeleteHello! Thank you for the comment! You may leave your questions in the comments. Once we finish our A2 in may this year, we will answer them and work hard to post as many solutions to questions as we can. Thank you for your patience!
DeleteThank you soo muchhh for these! truly life saving! My paper 1 exam is in a few days and I don't know what I'd be doing without these, thank you again so much! Just a question tho, for #40, where's the primary carbon with the double bond in structure 3 that will form the carbon dioxide? can't seem to notice this one
ReplyDeleteOh found it, never mind xD ... thank you once again though, very grateful for you guys
DeleteCan you explain s16 qp 12 q7.
ReplyDeleteFor Q#40, you may have drawn the '-COCH3' on the wrong side in the 2nd structure.
ReplyDeleteplease correct me if I'm wrong.
btw great blog, v.v helpful, can't thank you enough.
As a global Contract Research Organization (CRO), headquartered in New York, USA, Alfa Chemistry has served the pharmaceutical and biotechnology industries for eight years. Acid Red 57
ReplyDeleteI was reading some of your content on this website and I conceive this internet site is really informative ! Keep on putting up. physics
ReplyDeleteAlfa Chemistry employs more than 200 full time staff, of which approximate 80 are Ph.D. and M.S. chemists, specialized in synthetic chemistry, process optimization, and research.
ReplyDelete1-ethyl-2,3-dimethylimidazolium iodide
s02 p4 qno 4 c
ReplyDeleteThey needn't bother with any presentation. Pepsi are realized worldwide yet at the same time they burned through a huge number of dollars on changing their logo configuration to make an exceptional plan to individuals. This is one great why you should have your own logo plan. On the off chance that an organization, for example, Pepsi is offering significance to it, at that point it implies that it is something that you ought not overlook especially by independent companies. logo design service
ReplyDeletePositive site, where did u come up with the information on this posting?I have read a few of the articles on your website now, and I really like your style. Thanks a million and please keep up the effective work. types of logos
ReplyDeletecan you please explain Q# 39 of 9701/12/O/N/15 .
ReplyDeleteYes plz!
DeleteYou have it placed in boxes and put into the storage compartment of your vehicle and as far as possible home you trust you don't hit a knock. glass bottle wholesale price
ReplyDeleteThank you so much. It helped me a lot.But can you please publish past papers solution of recent years, like winter 2017 or summer 2019,2020
ReplyDeleteA drug organization is only scientists, as a matter of fact. A drug organization after it begins creating a gain from the drugs they delivered in any case needs to financial plan for an extremely durable exploration group to make future drug benefits.
ReplyDeletehow to buy cocaine online